How To Calculate Molarity Of Stock Solution
Enter 10 into the concentration box and select the correct unit. The standard formula is c mv.
:max_bytes(150000):strip_icc()/606823-calculate-molarity-of-a-solution-FINAL-5b7d7e15c9e77c0050355d4e.png) Learn How To Calculate Molarity Of A Solution
Learn How To Calculate Molarity Of A Solution
how to calculate molarity of stock solution
how to calculate molarity of stock solution is a summary of the best information with HD images sourced from all the most popular websites in the world. You can access all contents by clicking the download button. If want a higher resolution you can find it on Google Images.
Note: Copyright of all images in how to calculate molarity of stock solution content depends on the source site. We hope you do not use it for commercial purposes.
Enter 19713 into the molecular weight mw box.
How to calculate molarity of stock solution. Mass from volume concentration. The initial molarity m 1 comes from the stock solution and is therefore 15 m. In chemistry a solutions concentration is how much of a dissolvable substance known as a solute is mixed with another substance called the solvent.
What is the mass of compound required to make a 10 mm stock solution in 10 ml of water given that the molecular weight of the compound is 19713 gmol. Calculate mass required for molar solution. The mass molarity calculator tool calculates the mass of compound required to achieve a specific molar concentration and volume.
To calculate molarity divide the number of moles of solute by the volume of the solution in liters. Because your browser does not support javascript probably because javascript is disabled in an options or preferences dialog the calculators below wont work. If you dont know the number of moles of solute but you know the mass start by finding the molar mass of the solute which is equal to all of the molar masses of each element in the solution added together.
The solution dilution calculator tool calculates the volume of stock concentrate to add to achieve a specified volume and concentration. Desired volume and. Calculating the concentration of a chemical solution is a basic skill all students of chemistry must develop early in their studies.
The final molarity is the one you want in your final solution which is 0200 m. You can also calculate the mass of a substance needed to achieve a desired molarity. An example of a molarity calculation using the tocris molarity calculator.
Concentration refers to the amount of solute that is dissolved in a solventwe normally think of a solute as a solid that is added to a solvent eg adding table salt to water but the solute could easily exist in another phase. To dilute a solution of known molarity please use the solution dilution calculator. Calculate the molarity of a solution prepared by dissolving 237 grams of kmno 4 into enough water to make 750 ml of solution.
This example has neither the moles nor liters needed to find molarity so you must find the number of moles of the solute first. This article will provide you with the molarity definition and the molarity formula. The final volume is the one you want for your final solution 500.
Stock solution molarity and volume and 2 represents the diluted conditions ie. This molarity calculator is a tool for converting the mass concentration of any solution to molar concentration or recalculating the grams per ml to moles. Ml which is equivalent to 0500 l.
Using these known values you can calculate the initial volume v 1. How to calculate the concentration of a solution. The calculator uses the formula m 1 v 1 m 2 v 2 where 1 represents the concentrated conditions ie.
Molarity And Solution Units Of Concentration
 4 Ways To Calculate Molarity Wikihow
4 Ways To Calculate Molarity Wikihow
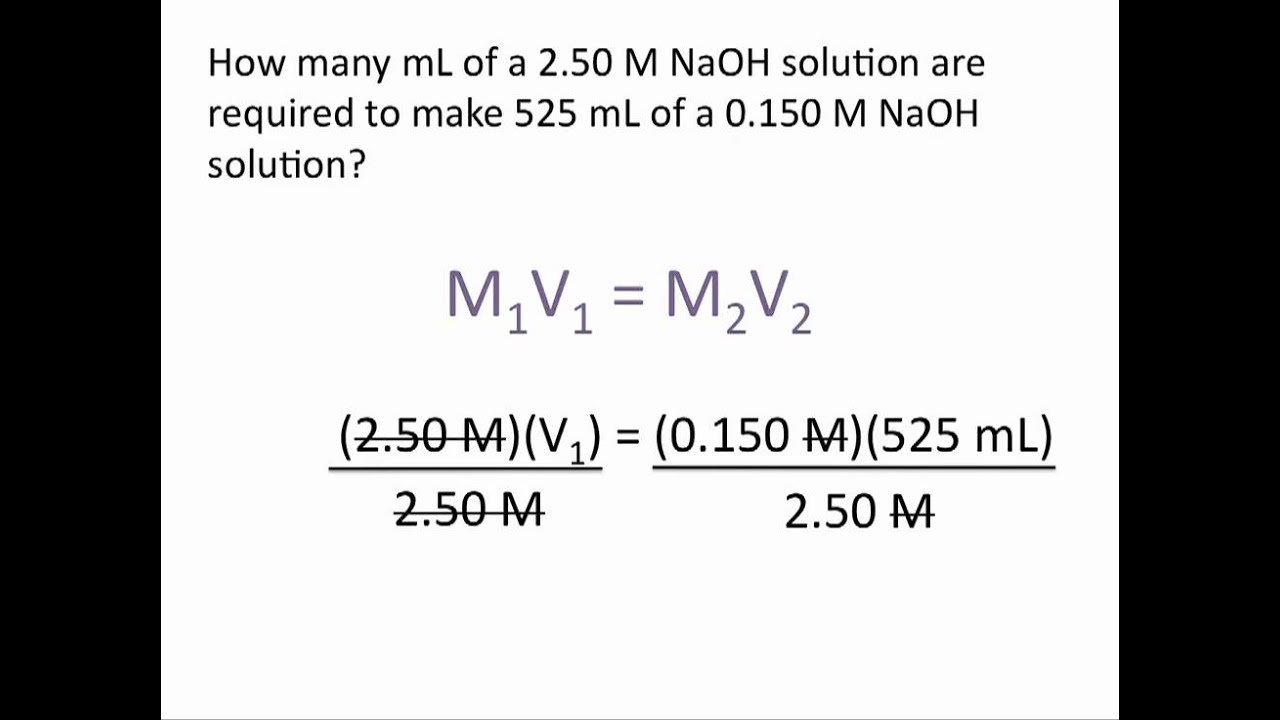 Dilution Problems Chemistry Tutorial
Dilution Problems Chemistry Tutorial
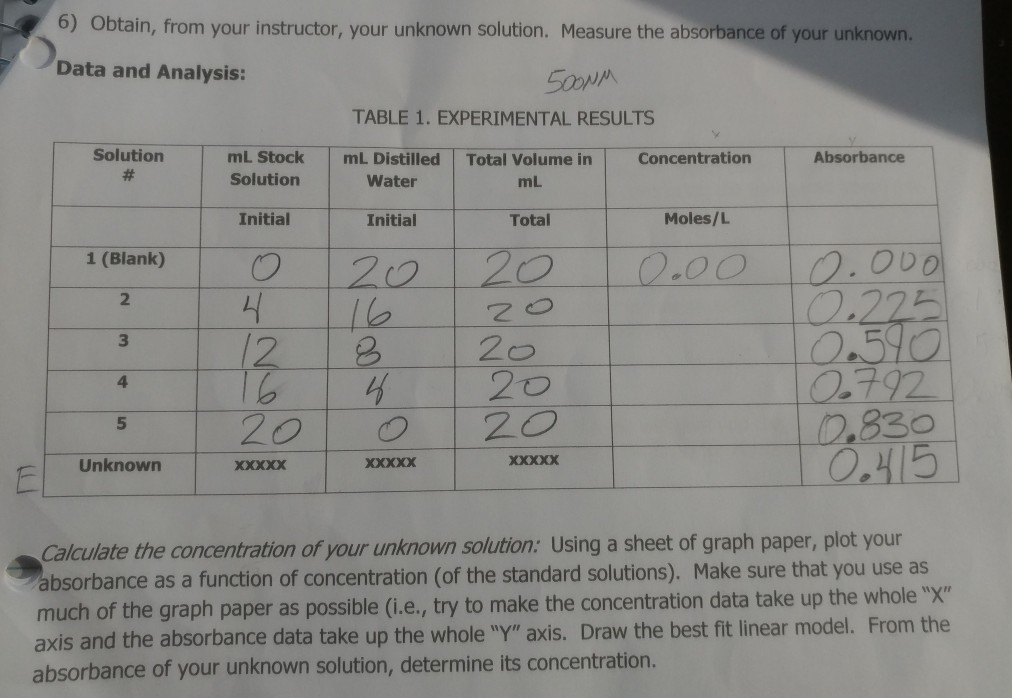

 Calculating Dilution Of Solutions
Calculating Dilution Of Solutions
 Solution Dilution Calculator Sigma Aldrich
Solution Dilution Calculator Sigma Aldrich
 Dilution Problems Chemistry Molarity Concentration Examples Formula Equations
Dilution Problems Chemistry Molarity Concentration Examples Formula Equations

